For centuries, arsenic has been used as a drug and as a poison.
GROUNDWATER ARSENIC Ś For centuries, arsenic has been used as a drug and as a poison.
Arsenic is an element with atomic number 33 and atomic weight 74.92. It exists throughout the earthÆs crust and is the 20th abundant element in nature.
For centuries, arsenic has been used as a drug and as a poison. Arsenic is thought to exert its toxicity by combining with certain enzymes and thereby interfering with cellular metabolism.
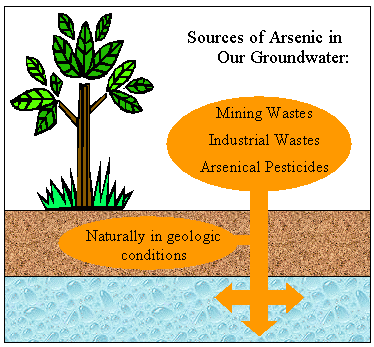
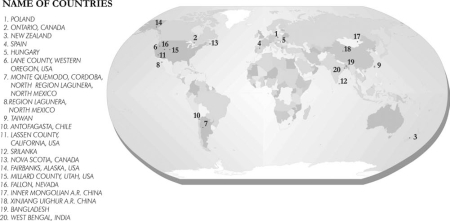
Groundwater arsenic contamination and sufferings of people have been reported in 20 countries in different parts of the world (Fig. 1). The magnitude is considered highest in four Asian countries, and the severity order is Bangladesh > West BengalŚIndia > P.R. China > Taiwan.
Fig. 1 Shows groundwater arsenic incidents round the world:
Toxicity
The toxicity of arsenic and its compounds is highly variable. Organic forms appear to have a lower toxicity than inorganic forms of arsenic.
Research has shown that arsenites (trivalent forms) have a higher acute toxicity than arsenates (pentavalent forms). The acute minimal lethal
dose of arsenic in adults is estimated to be 70 to 200 mg or 1 mg/kg/day. Most reported arsenic poisonings are not caused by elemental arsenic,
but by one of arsenics compounds, especially arsenic trioxide, which is approximately 500 times more toxic than pure arsenic. Symptoms include
violent stomach pains in the region of the bowels; tenderness and pressure; retching; excessive saliva production; vomiting; sense of dryness
and tightness in the throat; thirst; hoarseness and difficulty of speech; the matter vomited, greenish or yellowish, sometimes streaked with blood; diarrhea; tenesmus;
sometimes excoriation of the anus; urinary organs occasionally affected with violent burning pains and suppression; convulsions and cramps; clammy sweats; lividity of the extremities;
countenance collapsed; eyes red and sparkling; delirium; death. Some of these symptoms may be absent where the poisoning results from inhalation, as of arseniuretted hydrogen.
Symptoms of arsenic poisoning start with mild headaches and can progress to lightheadedness and usually, if untreated, will result in death.
Arsenic poisoning can lead to a variety of problems, from skin cancer to keratoses of the feet.
Chronic exposure to inorganic arsenic may lead to cutaneous hyperpigmentation.
Pathophysiology
Arsenic disrupts ATP production through several mechanisms. At the level of the citric acid cycle, arsenic inhibits pyruvate dehydrogenase and by competing with phosphate it uncouples oxidative phosphorylation, thus inhibiting energy-linked reduction of NAD+, mitochondrial respiration, and ATP synthesis. Hydrogen peroxide production is also increased, which might form reactive oxygen species and oxidative stress. These metabolic interferences lead to death from multi-system organ failure, probably from necrotic cell death, not apoptosis. A post mortem reveals brick red colored mucosa, due to severe hemorrhage. Although arsenic causes toxicity, it can also play a protective role.
Diagnosis
There are tests available to diagnose poisoning by measuring arsenic in blood, urine, hair, and fingernails. The urine test is the most reliable test for arsenic exposure within the last few days. Urine testing needs to be done within 24¢48 hours for an accurate analysis of an acute exposure. Tests on hair and fingernails can measure exposure to high levels of arsenic over the past 6¢12 months. These tests can determine if one has been exposed to above-average levels of arsenic. They cannot predict, however, whether the arsenic levels in the body will affect health.
Hair is a potential bioindicator for arsenic exposure due to its ability to store trace elements from blood. Incorporated elements maintain their position during growth of hair. Thus for a temporal estimation of exposure, an assay of hair composition needs to be carried out with a singe hair which is not possible with older techniques requiring homogenization and dissolution of several strands of hair. This type of biomonitoring has been achieved with newer microanalytical techniques like Synchroton radiation based X ray fluorescence (SXRF) spectroscopy and Microparticle induced X ray emission (PIXE).The highly focused and intense beams study small spots on biological samples allowing analysis to micro level along with the chemical speciation. In a study, this method has been used to follow arsenic level before, during and after treatment with Arsenious oxide in patients with Acute Promyelocytic Leukemia.
Treatment
Chemical and synthetic methods are now used to treat arsenic poisoning. Dimercaprol and dimercaptosuccinic acid are chelating agents which sequester the arsenic away from blood proteins and are used in treating acute arsenic poisoning. The most important side-effect is hypertension. Dimercaprol is considerably more toxic than succimer. In the journal Food and Chemical Toxicology, Keya Chaudhuri of the Indian Institute of Chemical Biology in Kolkata, and her colleagues reported giving rats daily doses of arsenic in their water, in levels equivalent to those found in groundwater in Bangladesh and West Bengal. Those rats which were also fed garlic extracts had 40 percent less arsenic in their blood and liver, and passed 45 percent more arsenic in their urine. The conclusion is that sulfur-containing substances in garlic scavenge arsenic from tissues and blood. The presentation concludes that people in areas at risk of arsenic contamination in the water supply should eat one to three cloves of garlic per day as a preventative.
